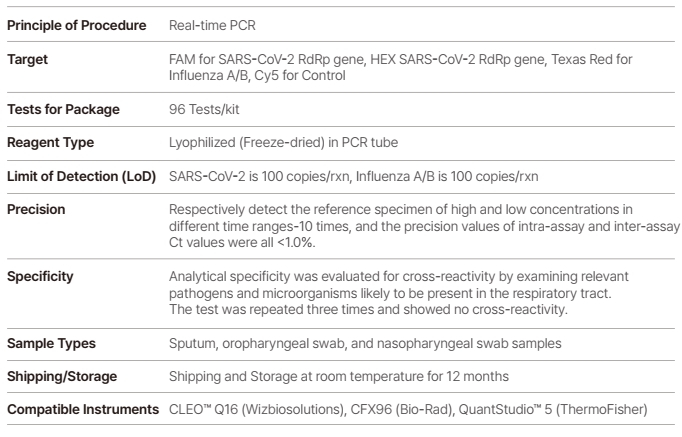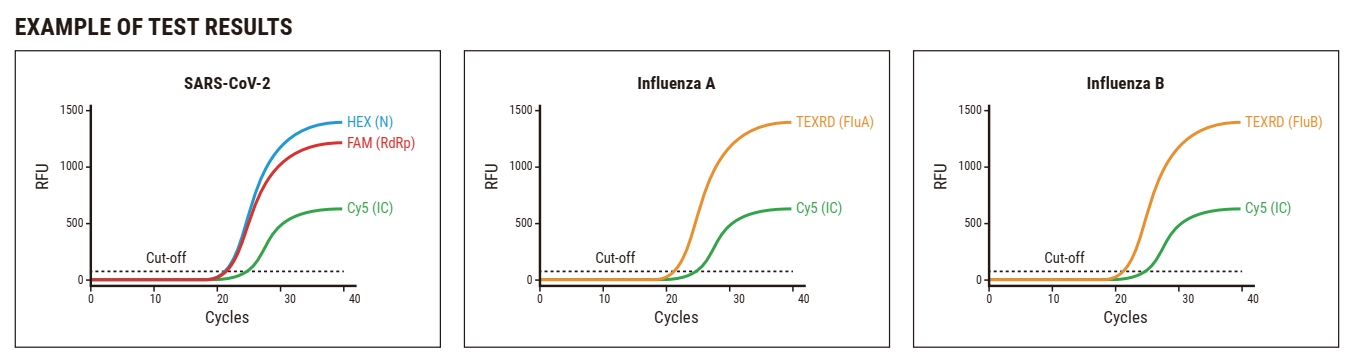



WizDx™ SARS-CoV-2/Flu CrystalMix PCR Kit is a Multiplex real-time RT-PCR test intended for the simultaneous qualitative detection f RNA from the SARS-CoV-2, influenza viruses in the nasopharyngeal swab, oropharyngeal swabs, and sputum from patients.
WizDx™ SARS-CoV-2/Flu CrystalMix PCR Kit combines all the reagents necessary for successful one-step real-time RT-PCR in a convenient individually aliquot and lyophilized in an 8-strip qPCR tube.
MFDS License No. : IVD-22-856
Download